Hydrogen facts - Hydrogen
Breadcrumb
Banner Facts
Facts
The "colors" of hydrogen, its sustainable production and its role in the energy transition.
Colors of H2
Hydrogen: Colorful variety
Not all hydrogen is the same - colors play a decisive role in its classification.
Hydrogen produced in a CO2-neutral manner is the means of choice for achieving climate neutrality in industrial processes and production as well as in logistics processes and public transport.
-
Green hydrogen...
-
Grey hydrogen...
-
Blue hydrogen...
-
Turquoise hydrogen...
-
Pink hydrogen...
-
Orange hydrogen...
... also called renewable hydrogen, is produced by electrolysis of water, whereby the electricity required for this must come exclusively from renewable energy sources. This makes its production CO2-neutral.
... is obtained from fossil fuels, usually natural gas. The steam reforming process used in this process also produces CO2, which escapes unused into the atmosphere. The production of one ton of hydrogen causes about ten tons of CO2.
... is basically gray hydrogen, whereby the CO2 is captured during production and permanently stored in geological formations (Carbon Capture and Storage, CCS) or used in other applications, e.g. in building materials (Carbon Capture and Utilization, CCU). Since the CO2 is not released into the atmosphere, the production of blue hydrogen is considered CO2-neutral.
... comes from the thermal cracking of methane (methane pyrolysis). Instead of CO2, solid carbon is produced. For turquoise hydrogen to be considered CO2-neutral, the heat supply of the high-temperature reactor must come from renewable energy sources and the carbon must be permanently sequestered - unless the methane comes from biogenic sources.
... is also produced from the electrolysis of water, but the electricity used for this comes from nuclear power plants. Since no CO2 emissions are produced, the production of pink hydrogen is considered climate-neutral.
... is generated from biomass or with electricity from waste management, for example from waste incineration plants or biogas plants.
Hydrogen production: electrolysis of water Part 1/2
Hydrogen production: electrolysis of water
Whether as an energy storage medium, fuel or basic material for chemical processes – green hydrogen is considered to be the ultimate energy carrier of the future. Low-temperature water electrolysis is used to produce clean hydrogen, and a distinction is made between three basic processes:
Accordeon - Hydrogen production: electrolysis of water
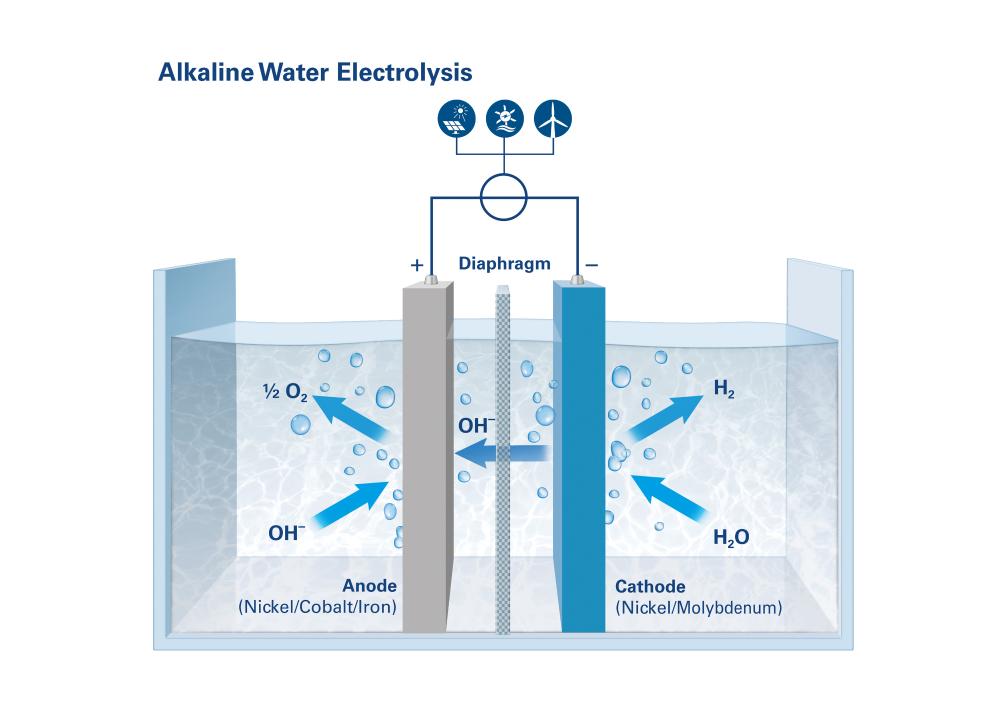
Alkaline water electrolysis (AWE)
AWE – the workhorse of water electrolysis methods
In alkaline water electrolysis (AWE), two electrodes are immersed in a liquid electrolyte, which is usually a highly concentrated aqueous potassium hydroxide solution. The anode and cathode sections of the electrolytic cell are separated by a diaphragm that provides for the transport of hydroxide ions between the anode and the cathode. The diaphragm must also isolate electrically and be impermeable to the product gases. The water molecules split into hydrogen and hydroxide ions at the cathode. The hydrogen rises as a gas, while the hydroxide ions migrate through the alkaline solution to the anode, where they react into water and oxygen. AWE is a robust technology that can operate with relatively inexpensive electrolytic cell materials. The catalysts responsible for driving the reactions contain nickel, cobalt and iron, for example, while other cell components are made of stainless steel.
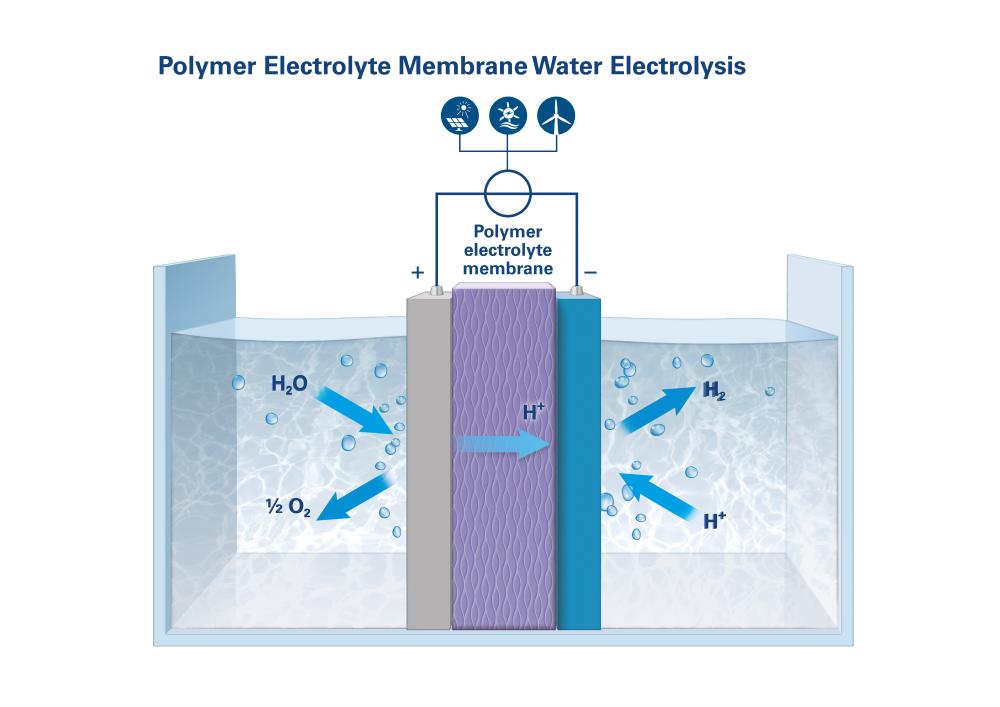
Polymer electrolyte membrane water electrolysis (PEMWE)
PEMWE – compact, dynamic and without external seals
In polymer electrolyte membrane water electrolysis (PEMWE), the membrane serves as more than just a separation wall. It also replaces the electrolytic fluid itself, because it is made of an ion-conducting polymer through which singly charged protons can migrate. The electrodes are placed in direct contact with the membrane. The water to be split flows to the anode, while the released hydrogen ions migrate from the anode through the membrane to the cathode side, where they bond with the hydrogen molecules. PEM electrolyzers operate at higher current densities than AWE systems and can also respond to load changes faster. Other advantages include their more compact design, dynamic operation and the ability to produce hydrogen under high pressure without external seals. This requires more expensive materials: platinum and iridium for the catalysts, and titanium for the electrolytic cells.
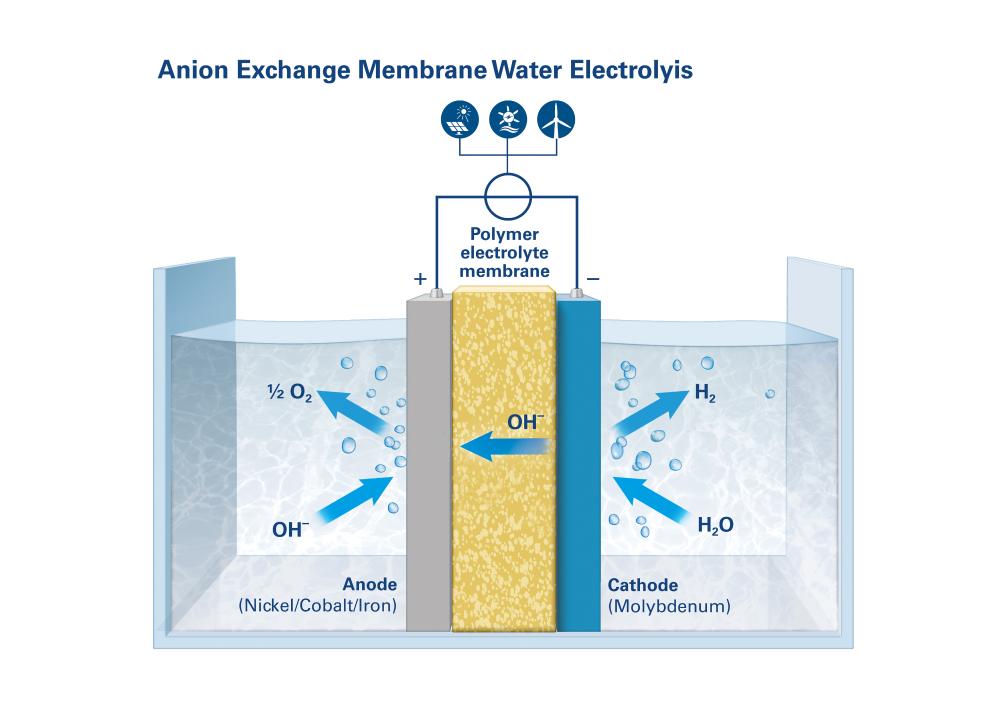
Anion exchange membrane water electrolysis (AEMWE)
AEMWE – a method with future potential
A third method, anion exchange membrane water electrolysis (AEMWE), is currently under development and holds potential for the future, because it combines the benefits of the two established technologies: inexpensive, readily available materials in a compact unit that produces hydrogen under high pressure.
Hydrogen production: electrolysis of water Part 2/2
For industrial hydrogen production, alkaline water electrolysis and polymer electrolyte membrane water electrolysis have already become established. In a direct comparison, the latter offers numerous advantages, which is why Messer prefers the PEMWE process.
In addition to the two established processes, the industrial use of anion exchange membrane water electrolysis (AEMWE) and high-temperature electrolysis called solid oxide electrolysis (SOEL) are currently being investigated.
Hydrogen policy: Defined climate targets
Hydrogen policy: Defined climate targets
Efforts are being made worldwide to counter the progressive warming of the earth's atmosphere and the associated climate change.
The European Union wants to become the first climate-neutral continent. Specifically, the European "Green Deal" envisages no more net greenhouse gas emissions by 2050. Germany plans to achieve climate neutrality in 2045. The USA wants to halve emissions of climate-damaging CO2 by 2030 compared to 2005 and be climate-neutral by 2050 at the latest. China is also committed to the goals of the Paris climate agreement.
Since 2019, the family-owned company Messer has been aligning itself with the UN Sustainable Development Goals. Through better utilization of existing production facilities and targeted projects that sustainably increase the energy efficiency of the plants, the specific energy consumption of Messer's own air separation plants is to be reduced further and further. To this end, Messer has set itself the target of reducing energy consumption by 0.7 percent per year by 2025; in total, this amounts to a reduction of almost 3.5 percent. In addition, Messer is making a further, significant contribution to decarbonization through the increasing use of renewable forms of energy.
In Europe, the European Commission's hydrogen strategy (2020) plays an important role in the decarbonization of transport and industry. It aims to create 6 GW of electrolysis capacity by 2024 and 40 GW of electrolysis capacity by 2030. The so-called 'Fit for 55' package of measures (2021) proposes, among other things, targets for the use of renewable hydrogen: 2.6% in transport and 50% in industry by 2030. The demand for hydrogen is to be met mainly by water electrolysis.
Rising EUA prices, stricter framework conditions in CO2 emissions trading, and incentives to switch to clean drive systems, including in local public transport, are further criteria for investment decisions in the direction of climate neutrality.
To support industry and companies on this path, Messer is ready with application technology expertise and products - including clean hydrogen. Through our presence in Europe, the USA and Asia, we are also familiar with the respective government programs to promote clean hydrogen projects and provide support in taking advantage of the associated opportunities.
